Presentation
What is Minerva?
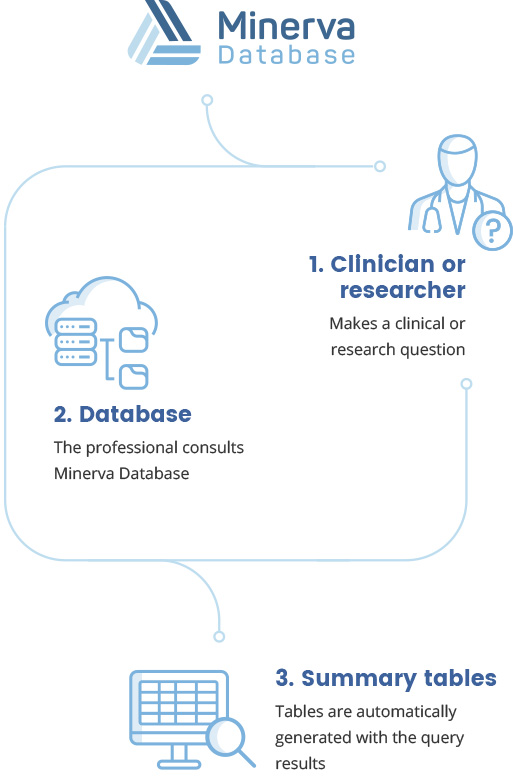
Minerva is a database of clinical trials with patients with attention deficit hyperactivity disorder (ADHD) that allows for quick queries, reviews and meta-analyses as the researcher skips the study identification and extraction stages of data.
Minerva is a database containing comprehensive information on all randomized clinical trials investigating the effects of medications in patients with attention deficit hyperactivity disorder (ADHD). We have identified clinical trials investigating the efficacy and/or safety of the pharmacological or psychological treatment of ADHD using multiple information sources. We have extracted administrative information, study design, characteristics of the patients, results and the risk of bias of all clinical trials (for detailed information on Minerva see Database design).
The information is stored in such a way that summary tables of clinical trials can be generated automatically. This information can also be easily exported for statistical analysis using common statistical analysis and meta-analysis software such as STATA, SPSS, SAS, CMA, R, Matlab, Python, Excel, or Review Managers.


Minerva in figures
Currently, Minerva contains information on almost 500 randomized clinical trials (RCTs) published in more than 1000 scientific articles, documents from regulatory agencies and pharmaceutical industry, and RCTs registries such as clinicaltrials.gov or eudraCT.eu.
How does Minerva Database help its users?

Efficiency
Save time and money as the use of Minerva database allows to skip study identification and data extraction.

Exhaustiveness
The database contains comprehensive information on the design, patients, interventions, and results of each clinical trial.

Validity of information
The information available in Minerva Database has been used in numerous published studies.

